The IR range of the spectrum extends from 1 m the long-wavelength end of the visible region to 1000 m in the microwave region. The major part of this assignment is an analysis of the infrared vibration-rotation spectrum of HCl in terms of the theoretical model discussed above First try a regression fit of the equation.
D.

. In the laboratory rather than prepare and analyze a fresh sample of HCl we will examine and manipulate a spectrum that has already been recorded and saved. Suggest one reason why HCl has only one peak. THE HIGH-RESOLUTION INFRARED SPECTRUM OF HCl J.
Cm 2 566805 3 834698 4 1092311 5 1339655 PrecautionsNotes 1. There are two tables grouped by frequency range and compound class. 5 Calculate the force constant for the HCl bond.
For I the moment of inertia of the HCl molecule. Infrared spectroscopy of HCL spectrum and animations of molecular motion. These values represent 10 error.
Simplest vibrating diatomic model is a harmonic oscillator described by. The table lists IR spectroscopy frequency ranges appearance of the vibration and absorptions for functional groups. As for the force constant the literature value for HCl is 481000 dynescm.
GAS 200 mmHg DILUTED TO A TOTAL PRESSURE OF 600 mmHg WITH N2. The High Resolution Infrared Spectrum of HCl The High Resolution Infrared Spectrum of HCl Authors J L Doménech 1 B J Drouin 2 J Cernicharo 3 V J Herrero 4 I Tanarro 4 Affiliations 1 Molecular Physics Department Instituto de Estructura de la Materia IEM-CSIC Serrano 123. GAS 200 mmHg N2 ADDED TOTAL PRESSURE 600 mmHg.
For IR absorption to take place the stretch of bond should introduce a difference in the dipole moment of the molecule which manifests as the different energy levels. 4 Using 167379 x 10-27 kg and 580752 x 10-26 kg for the masses of individual atoms of hydrogen and chlorine respectively compute the reduced mass µ and bond length r e in Angstroms and nm for HCl from I µ r e 2. Use the infrared vibrational spectrum of HCl and DCl to obtain the following.
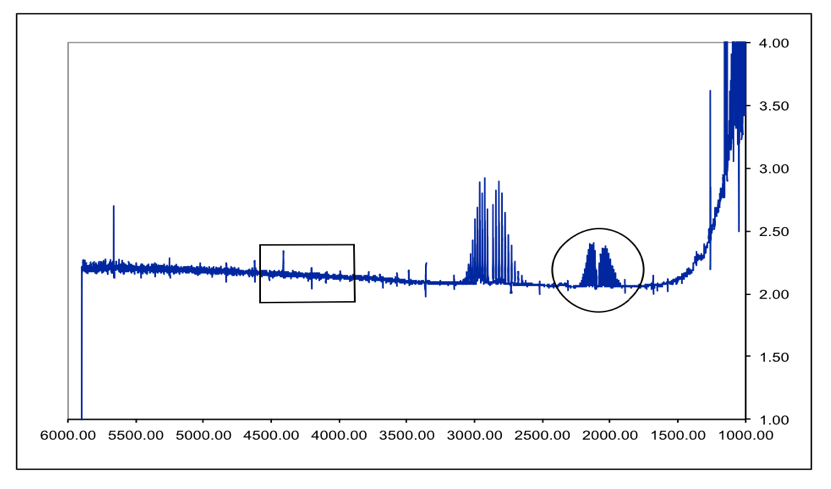
The infrared absorption spectrum of the HCl molecule is measured using a Fourier-transform infrared FTIR spectrometer. For simple diatomic molecules the only possible mode of vibration is. Location HCl spectrum DCl spectrum Mirror.
The IR Spectrum Table is a chart for use during infrared spectroscopy. Tanarro1 1 Molecular Physics Department Instituto de Estructura de la Materia IEM-CSIC Serrano 123 E-28006 Madrid Spain. IR Spectrum Table by Frequency Range.
34 Chapter 6 Analysis of the Infrared Spectrum of HCl Band origins for the HCl infrared transitions. Top References Notes Data compiled by. 𝜈 a 0 a 1 m Use a residual plot to show that it is necessary to add a term a 2 m2to the model.
Hydrogen Chloride HCl Hydrogen Chloride HCl HCl is a very simple example for demonstrating how molecules absorb radiation. Jldomenechcsices 2 Jet Propulsion Laboratory California Institute of Technology 4800 Oak Grove Drive Pasadena. D OM EN EC H Molecular Physics Department Instituto de Estructura de la Materia IEM-CSIC Serrano 123.
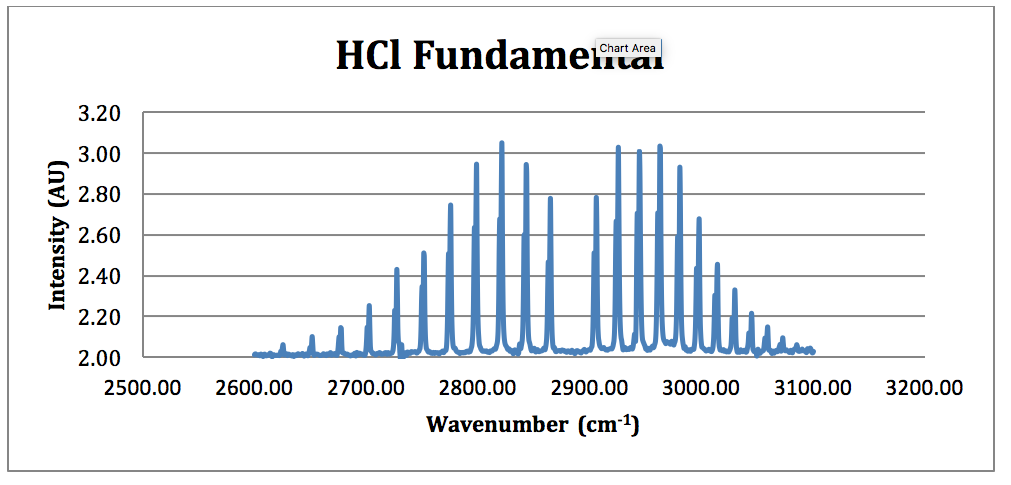
Simplest rotating diatomic model is the rigid rotor or dumb-bell model which can be pictured as two masses joined by a rigid weightless rod and described by. Does this have something to do with fact that peaks in IR spectroscopy represent areas of the spectrum where specific bond vibrations occur and therefore since HCl has a single bond it. In this experiment we measure the infrared IR vibrational spectrum of a linear diatomic HCl molecule in the gas phase with rotational resolution ie with the rotational fine structure.
The calculated values for this were 527667 527781 522901 and 521422 dynescm. HCl and DCl IR absorption spectra Datasets PDF files of peak-labeled high resolution. This value is actually relatively good for the instrument we were using and the sample purities given.
DIGITIZED BY NIST FROM HARD COPY FROM TWO SEGMENTS. THE HIGH RESOLUTION INFRARED SPECTRUM OF HCl J. The spectra from several isotopes of HCl are an-alyzed for common information about the molecular bond and for variations arising.
Spectra were collected on a Nicolet Nexus 670 FR-IR in a 10-cm gas cell at a pressure of 20 Torr. The chlorine isotope peaks are resolved to baseline. Because Cl is much more electronegative than H HCl has a dipole moment.

Ft Ir Spectra Of Lidocaine Hcl Hp B Cd 1 1 And 1 2 Lidocaine Download Scientific Diagram

Infrared Spectrometric Rotational And Vibrational Analysis Of Hcl And Dcl Caroline Frank
Spectroscopy And Molecular Structure Hci Dci

Atr Ftir Spectra Of The Standards Of A Cocaine Base And B Cocaine Download Scientific Diagram

Ir Spectrum Of Gemcitabine Hcl Download Scientific Diagram

Infrared Spectrometric Rotational And Vibrational Analysis Of Hcl And Dcl Caroline Frank
Spectroscopy And Molecular Structure Hci Dci

Ketamine Standard Ftir Spectra Download Scientific Diagram

Ft Ir Spectra Of A Fexofenadine B A Mixture Of Fexofenadine And R Download Scientific Diagram

Measured Hcl Spectrum At The Mid Wave Infrared Notice The P And R Download Scientific Diagram

Ir Spectrum Of Gemcitabine Hcl Download Scientific Diagram
.jpg)
Analyzing The Gas Phase Spectrum Of Hydrogen Chloride With Ft Ir

Experiment 9 Rotational Vibrational Spectroscopy Introduction

Atr Ftir Spectra Of The Standards Of A Cocaine Base And B Cocaine Download Scientific Diagram
